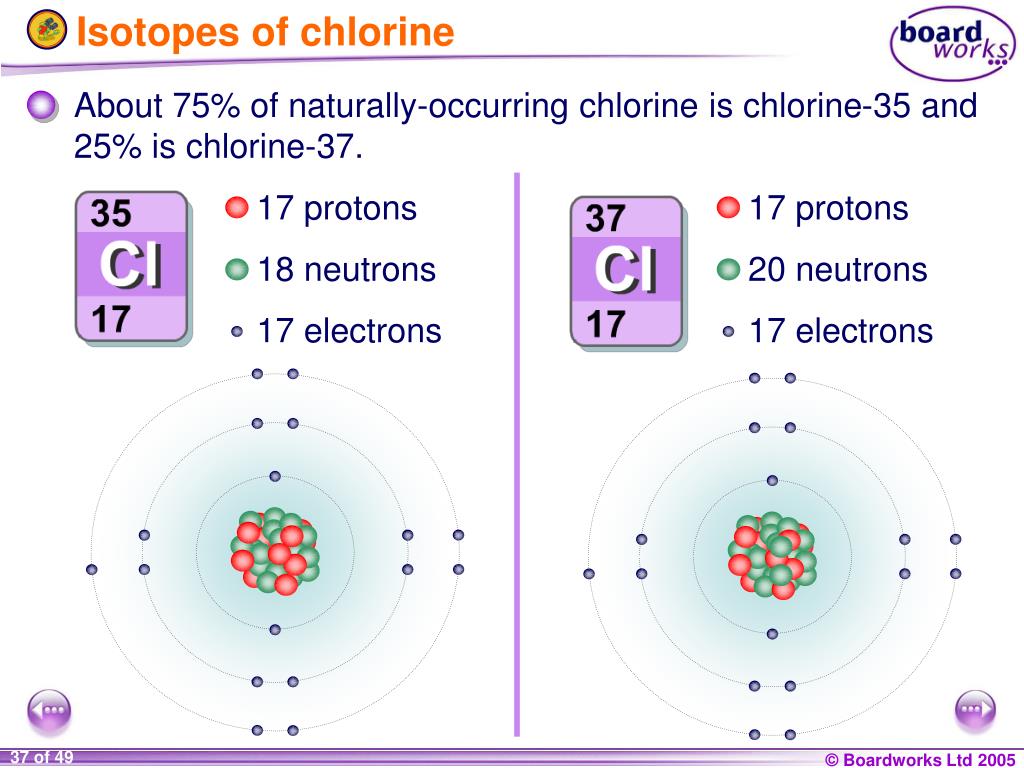
Isotopes Of Chlorine-35 And 37 Ratio . Isotopes are the atoms of the same element having different. There are two stable isotopes, 35 cl. The atomic weight of chlorine given on the periodic table is 35.47 u. Atomic mass of chlorine is 35.5. This indicates the ratio of c l − 37 and c l − 35 is approximately: 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. Different isotopes have different relative. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. Chlorine exists in two isotopic forms. Chlorine has two stable isotopes: The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: Isotopes of chlorine with mass number 35 and 37 exist in ratio?
from www.slideserve.com
The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: This indicates the ratio of c l − 37 and c l − 35 is approximately: Chlorine exists in two isotopic forms. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Atomic mass of chlorine is 35.5. Isotopes of chlorine with mass number 35 and 37 exist in ratio? Isotopes are the atoms of the same element having different. There are two stable isotopes, 35 cl. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. The atomic weight of chlorine given on the periodic table is 35.47 u.
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233
Isotopes Of Chlorine-35 And 37 Ratio 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two stable isotopes, 35 cl. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. Isotopes of chlorine with mass number 35 and 37 exist in ratio? Chlorine exists in two isotopic forms. Isotopes are the atoms of the same element having different. This indicates the ratio of c l − 37 and c l − 35 is approximately: Different isotopes have different relative. Atomic mass of chlorine is 35.5. Chlorine has two stable isotopes: The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The atomic weight of chlorine given on the periodic table is 35.47 u.
From www.researchgate.net
Relative variations of chlorine/bromine and carbon isotope ratios (Δ 37 Isotopes Of Chlorine-35 And 37 Ratio Atomic mass of chlorine is 35.5. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. Chlorine exists in two isotopic forms.. Isotopes Of Chlorine-35 And 37 Ratio.
From www.doubtnut.com
Chlorine has two isotope ""(17)Cl^(35)Cl^(35) . The atomic weight of Isotopes Of Chlorine-35 And 37 Ratio 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine has two stable isotopes: This indicates the ratio of c l − 37 and c l − 35 is approximately: Chlorine exists in two isotopic forms, c l − 37 and c. Isotopes Of Chlorine-35 And 37 Ratio.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Isotopes Of Chlorine-35 And 37 Ratio Atomic mass of chlorine is 35.5. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine has two stable isotopes: Chlorine exists in two isotopic forms. This indicates the ratio of c l − 37 and c l − 35 is approximately:. Isotopes Of Chlorine-35 And 37 Ratio.
From present5.com
Isotopes Atoms of the same element with Isotopes Of Chlorine-35 And 37 Ratio Chlorine has two stable isotopes: Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Atomic mass of chlorine is 35.5. Different. Isotopes Of Chlorine-35 And 37 Ratio.
From www.meritnation.com
The isotopes of chlorine with mass numbers 35 and 37 exist in the ratio Isotopes Of Chlorine-35 And 37 Ratio 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Atomic mass of chlorine is 35.5. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The isotopes of chlorine. Isotopes Of Chlorine-35 And 37 Ratio.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic Isotopes Of Chlorine-35 And 37 Ratio 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine exists in two isotopic forms, c l − 37. Isotopes Of Chlorine-35 And 37 Ratio.
From brainly.in
If chlorine atom is available in the form of two isotopes 35cl 75and Isotopes Of Chlorine-35 And 37 Ratio Isotopes are the atoms of the same element having different. This indicates the ratio of c l − 37 and c l − 35 is approximately: Chlorine has two stable isotopes: The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: Atomic mass of chlorine is 35.5. 35 cl, along. Isotopes Of Chlorine-35 And 37 Ratio.
From www.researchgate.net
Chlorine/bromine isotope ratios (IR 37 Cl/ 35 Cl or 81 Br/ 79 Br) of Isotopes Of Chlorine-35 And 37 Ratio There are two stable isotopes, 35 cl. 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Different isotopes have different relative. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and. Isotopes Of Chlorine-35 And 37 Ratio.
From www.slideshare.net
Atomic Structure Part 2 Isotopes Of Chlorine-35 And 37 Ratio Isotopes of chlorine with mass number 35 and 37 exist in ratio? Isotopes are the atoms of the same element having different. The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for. Isotopes Of Chlorine-35 And 37 Ratio.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Isotopes Of Chlorine-35 And 37 Ratio Isotopes are the atoms of the same element having different. Chlorine has two stable isotopes: There are two stable isotopes, 35 cl. Atomic mass of chlorine is 35.5. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. 35 cl, along with 37 cl is one of the two. Isotopes Of Chlorine-35 And 37 Ratio.
From www.meritnation.com
The isotopes of chlorine with mass numbers 35 and 37 exist in the ratio Isotopes Of Chlorine-35 And 37 Ratio Chlorine exists in two isotopic forms. This indicates the ratio of c l − 37 and c l − 35 is approximately: Different isotopes have different relative. Chlorine has two stable isotopes: Isotopes of chlorine with mass number 35 and 37 exist in ratio? 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and. Isotopes Of Chlorine-35 And 37 Ratio.
From brainly.in
Cl35 and Cl37 are two isotopes of chlorine. If the average atomic Isotopes Of Chlorine-35 And 37 Ratio There are two stable isotopes, 35 cl. The isotopes of chlorine with mass numbers 35 and 37 whose average mass is 35.5 exist in the ratio of: Chlorine has two stable isotopes: Different isotopes have different relative. This indicates the ratio of c l − 37 and c l − 35 is approximately: Isotopes of chlorine with mass number 35. Isotopes Of Chlorine-35 And 37 Ratio.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Isotopes Of Chlorine-35 And 37 Ratio 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. This indicates the ratio of c l − 37 and c l − 35 is approximately: The atomic weight of chlorine given on the periodic table is 35.47 u. There are two stable isotopes, 35 cl. 35. Isotopes Of Chlorine-35 And 37 Ratio.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Isotopes Of Chlorine-35 And 37 Ratio There are two stable isotopes, 35 cl. The atomic weight of chlorine given on the periodic table is 35.47 u. Different isotopes have different relative. Isotopes are the atoms of the same element having different. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. 53 rows chlorine (17. Isotopes Of Chlorine-35 And 37 Ratio.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Isotopes Of Chlorine-35 And 37 Ratio Chlorine has two stable isotopes: Different isotopes have different relative. There are two stable isotopes, 35 cl. Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. The atomic weight of chlorine given on the periodic table is 35.47 u. Chlorine exists in two isotopic forms. The isotopes of. Isotopes Of Chlorine-35 And 37 Ratio.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Isotopes Of Chlorine-35 And 37 Ratio 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine has two stable isotopes: Chlorine exists in two isotopic forms, c l − 37 and c l − 35 but its atomic mass is 35.5. The atomic weight of chlorine given on. Isotopes Of Chlorine-35 And 37 Ratio.
From www.numerade.com
Section A Answer all the questions in the spaces provided (a) Chlorine Isotopes Of Chlorine-35 And 37 Ratio 35 cl, along with 37 cl is one of the two natural chlorine isotopes that are suitable for nmr spectroscopy ( 36 cl only occurs in trace. Chlorine has two stable isotopes: Isotopes are the atoms of the same element having different. There are two stable isotopes, 35 cl. Atomic mass of chlorine is 35.5. Isotopes of chlorine with mass. Isotopes Of Chlorine-35 And 37 Ratio.
From www.chegg.com
Solved Q15. Chlorine has two isotopes Cl35 and Cl37. The Isotopes Of Chlorine-35 And 37 Ratio Isotopes of chlorine with mass number 35 and 37 exist in ratio? This indicates the ratio of c l − 37 and c l − 35 is approximately: Isotopes are the atoms of the same element having different. Chlorine exists in two isotopic forms. There are two stable isotopes, 35 cl. 35 cl, along with 37 cl is one of. Isotopes Of Chlorine-35 And 37 Ratio.